 |
Principles & Structures |
|
The Fullymax lithium-ion polymer battery use of lithium cobalt oxide (which has superior Cycling properties at high voltage) as the positive electrode and a highly crystallized carbon as the negative electrode. It uses an organic solvent, optimized for the special carbon, as the electrolyte.
The Fullymax lithium-ion polymer battery is made of a substance capable of cyclic transfer of lithium-ions between two electrodes. The cell operates on the rocking chair principle that is charging and discharging cause lithium-ions to grockh back and forth between the positive and negative electrodes. This principle of operation enhance the cycle life of battery, since neither the anode nor the cathode material of the lithium-ion polymer battery essentially changes.
The chemical reactions for charge and discharge are as shown below:
1.The electrochemical reaction equation of positive electrode:
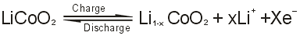
2.The electrochemical reaction equation of negative electrode:
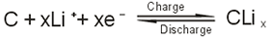
3.The electrochemical reaction equation as a whole:
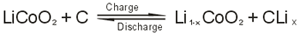
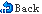
|